LANGUAGE:
- WHAT WE DO
- AI

- Industries

Content Services
- Technical Writing
- Training & eLearning
- Financial Reports
- Digital Marketing
- SEO & Content Optimization
Translation Services
- Video Localization
- Software Localization
- Website Localization
- Translation for Regulated Companies
- Interpretation
- Instant Interpreter
- Live Events
Testing Services
- Functional QA & Testing
- Compatibility Testing
- Interoperability Testing
- Performance Testing
- Accessibility Testing
- UX/CX Testing
Solutions
- Translation Service Models
- Machine Translation
- Smart Onboarding™
Our Knowledge Hubs
- Positive Patient Outcomes
- Modern Clinical Trial Solutions
- Future of Localization
- Innovation to Immunity
- COVID-19 Resource Center
- Disruption Series
- Patient Engagement
- Lionbridge Insights
Life Sciences
- Pharmaceutical
- Clinical
- Regulatory
- Post-Approval
- Corporate
- Medical Devices
- Validation and Clinical
- Regulatory
- Post-Authorization
- Corporate
Banking & Finance
Retail
Luxury
E-Commerce
Games
Automotive
Consumer Packaged Goods
Technology
Industrial Manufacturing
Legal Services
Travel & Hospitality
SELECT LANGUAGE:
In our latest series on COVID-19, Lionbridge experts offer perspectives on the ecosystem of clinical development and regulatory approvals during the pandemic and in the future.
With our scale, service excellence and suite of language, communication and technology solutions, Lionbridge is well positioned to support any accelerated efforts in bringing vaccines and lifesaving therapies to market.
The statistics in this article were updated on Monday, December 7, 2020.
The Race for a COVID-19 Vaccine
Put on your running shoes if you want to keep up with progress towards a vaccine for the COVID-19 virus. The development of vaccine candidates is happening with unprecedented speed, which makes the standard runway for developing new treatments look like a walk in the park. This race is happening with a rising human toll at its heels and requires action from all stakeholders in the global healthcare infrastructure. The pandemic has now resulted in over 1.5 million deaths and 66 million confirmed cases and the numbers are on the rise globally, according to the WHO.
At this point in the race, there are 1,157 trials in the global clinical pipeline addressing the SARS-CoV infection and at least 100 vaccines in development. On November 20, Pfizer and BioNTech broke the news that they would submit an Emergency Use Authorization (EUA) to the FDA for their vaccine candidate BNT162b2. And on November 25, Moderna announced that the European Commission has approved an advance purchase agreement for 80 million doses of their mRNA-1273 vaccine candidate.
Other candidates in the late stages of pipeline include AstraZeneca’s vaccine, known as AZD1222, which was developed in partnership with Oxford University, and the recombinant vaccine Ad5-nCoV, developed by Chinese based CanSino Biologics.
Improving Speed and Maintaining Quality
A sense of urgency and radical change are usually the most powerful response mechanisms to combat an emergent threat and certainly this pandemic is no different. Operational excellence and virtual trial conduct have been instrumental in the development of new COVID-19 vaccines, and the urgency and severity of the pandemic do not allow compromise on quality.
Regulatory authorities around the globe offer accelerated pathways for scientific advice and emergency use authorization. At the same time, they have emphasized that the investigational data must be sufficient in both quantity and quality to obtain a regulatory green light.
The FDA’s October guidance on Emergency Use Authorization for Vaccines to Prevent COVID-19 addresses the quality question head on. An EUA will only be issued if the agency determines that “the vaccine’s benefits outweigh its risks based on data from at least one well-designed Phase 3 trial that demonstrates the vaccine’s efficacy and safety in a clear and compelling manner.”
A constant balance between safety, efficacy and quality
In the current clinical pipeline for COVID-19 vaccines, we see extensive phase 3 trials recruiting up to 40,000 participants across hundreds of sites and initiating with impressive speed. Pfizer’s phase 2/3 safety and efficacy trial for their vaccine candidate (BNT162b2) was initiated only three months after the initiation of their phase 1/2 safety and immunogenicity trials. And only two hours after the FDA greenlight, Pfizer administered the first vaccine dose to the first trial participant.
In Russia, as of November 23, two vaccines had been approved. However, both of these were approved by the Russian Ministry of Health before entering phase 3. The August announcement from Vladimir Putin was received with great concern among vaccine experts who have called this regulatory acceleration “beyond stupid," as well as “really scary and really risky.”
The development of medicines is a constant balance between safety, efficacy and quality and the race for a vaccine is one where speed must go hand-in-hand with data integrity and reliability.
From Competition to Collaboration: A Race Against Time
During the pandemic, industry players that normally compete on time to market for new innovative therapies have shown an unusual collaboration effort. Proprietary technologies and expertise (normally heavily guarded inside the pharma enterprise) are now being shared to fast-track a COVID-19 vaccine.
One such partnership is the collaboration between GSK and Sanofi, two of the world’s largest vaccine manufacturers and long-term rivals. GSK has granted Sanofi access to its adjuvant technology which, in combination with Sanofi’s vaccine candidate, can enhance the immune response and reduce the necessary quantity of vaccine per dose. This enhancement will be critical given the extent of the pandemic and the partners’ commitment to the U.S. government to supply up to 100 million doses.
Running with the Pack: Concurrent Phase Implementation
This kind of development speed is record breaking.
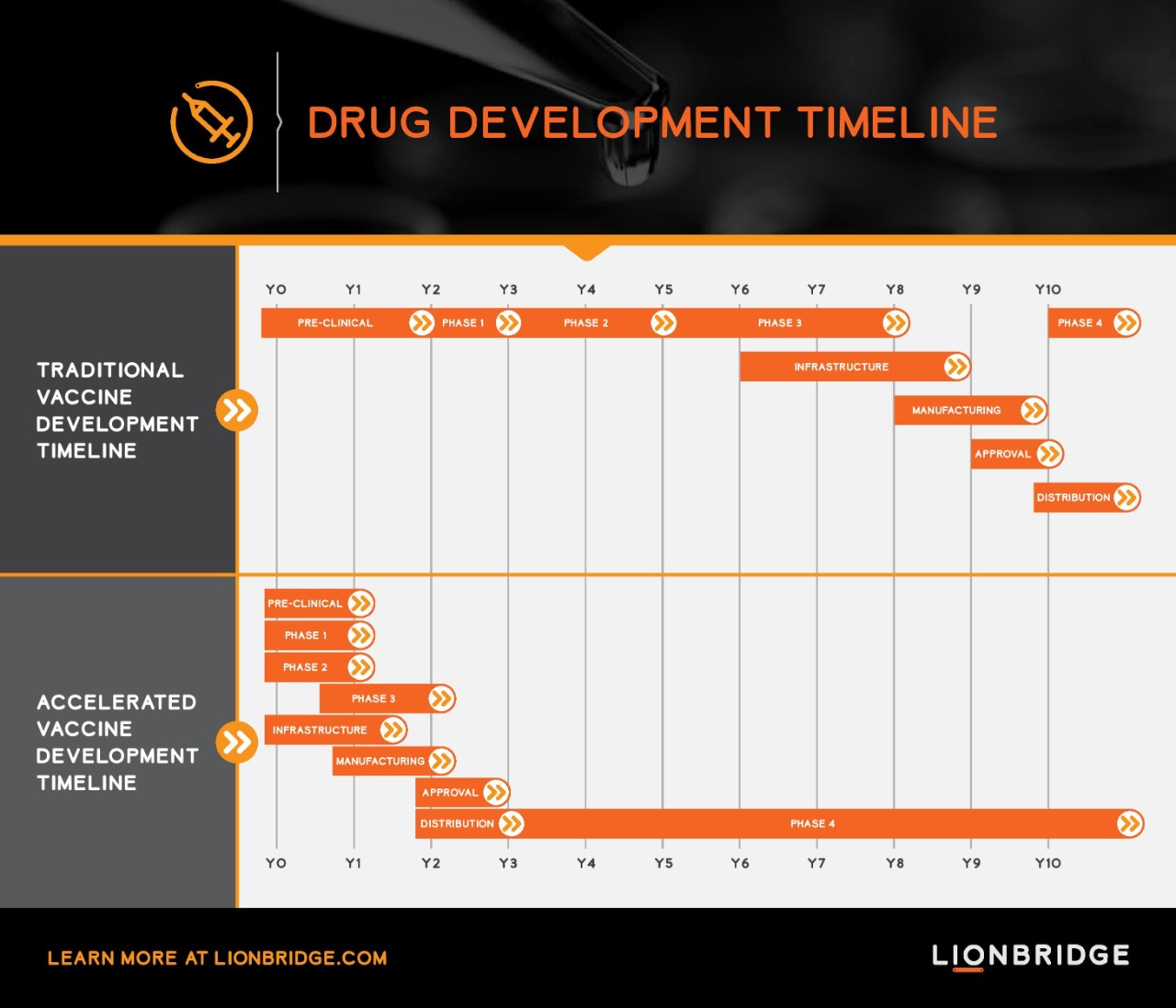
The development of a vaccine usually takes more than a decade, from discovery to regulatory licensing to the clinical development program that falls in sequential phases. The accelerated pathway we currently see for development of COVID-19 vaccines runs in a parallel execution mode. Seamless trial designs combining phase 1/2 and phase 2/3 are implemented to fast-track the development path. Trials include multiple outcome measures, multiple populations and more vaccine candidates.
For example, Pfizer tested four candidates in parallel in their phase 1/2 trial before selecting their lead candidate for phase 2/3 (BNT162b2). The phase 2/3 trial for BNT162b2 enrolled around 44,000 participants at 155 trial sites across U.S., Argentina, Brazil, Germany, South Africa and Turkey. The scale of this trial is not only extensive in number of participants and sites, but also in the diversity of the population enrolled in the trial. The trial was initiated enrolling adults in three different age groups up to 85 years of age and later expanded to include adolescents down to 12 years of age. In addition, the vaccine was tested in people who have chronic HIV and Hepatitis B or C. Pfizer and BioNTech—which are co-developers of the vaccine—expect to produce up to 50 million doses in 2020 and 1.3 billion doses by end of 2021.
COVID-19's Impact on the Future Service Model
The COVID-19 pandemic has demonstrated how a standard eight-year development track from pre-clinical phase to end of phase 3 can be reduced to just two years and enable a vaccine to reach the market in only three years. This kind of development speed is record breaking. It is the reason we now expect to see vaccines available by end of this year.

Will this unprecedented international effort across industry, regulators, governments and health organizations prompt a push for a faster execution for other lifesaving or rare therapies where no treatments are available to patients? Why shouldn't these patients benefit from the operational excellence that we have seen under the pandemic? The pandemic has not only shown that development and production can be fast-tracked by several years, but has also boosted the use of digital infrastructure and tools such as electronic diaries, telemedicine, smartphones and wearables that have helped accelerate trial execution.
Accelerated execution will impact the whole supply chain and service providers that will need to operate with increased speed and agility to meet the requirements of industry and regulatory authorities. For a language services provider, the acceleration of clinical studies and marketing authorizations will impact the service model and demand an athletic performance in delivery, resourcing and language capacity. The use of language assets, automation and scalability will become backbones of an expedited service suite along with diligent language quality assurance to manage risks.